- 9.35g of Al2O3 contains how many molecules?
- 0.253 moles of BN has a mass of…
- Calculate the number of moles of 8.46 x 1024 atoms of F.
- Calculate the number of moles of 4.35g of Cs2CO3.
- 0.692moles of CsH contains how many molecules?
- How many moles are in 14.49 g of CoSO4.
- What is the mass of 7.20 x 1023 atoms of Ne?
- 50.82 g of Cr2O3 has how many moles?
- 2.84 moles of N2H4 has how many molecules?
- 5.29 x 1024 molecules of HCl has a mass of…
- In a 30.0 g sample of Pb(NO3)2, Pb has a mass of 2.535 g, N a mass of 8.694 g, and O a mass of 18.768 g. What is the % composition?
- In a 25.0 g sample of Hg2Cl2, Hg has a mass of 3.755 g and Cl has a mass of 21.245 g. What is the % composition.
- In a 20.0 g sample of Ni(NO3)2, Ni has a mass of 3.066 g, Ni has a mass of 6.426 g, and O has a mass of 10.508 g. What is the % composition?
- What is the % composition of the following:
- KMnO4
- Sc2O3
- H2SeO4
- Cl3HSi
- NaBrO
- What volume of 0.075 M HCl is required to neutralize 100 mL of 0.01 M NaOH solution?
- What is the concentration of NaOH if 45.2 mL of 1.5 M H2SO4 is needed to neutralize 20.0 mL of NaOH: H2SO4 + 2NaOH ➝ Na2SO4 + 2H2O?
- Find the [H3O+] and pH of the following: 0.075 M HCl
- Find the [H3O+] and pH of the following: 0.122 M H2SO4
- Calculate the [H3O+] and pOH of the following solution: pH = 9.5
- Calculate the [H3O+] and pOH of the following solution: [OH-] = 3.9 x 10-12
- Find the [OH-] of the following solutions: pOH = 1.5, pH = 4.6, & [H3O+] = 2.5 x 10-9
- In the following chemical reactions, identify the acid, base, conjugate acid, and conjugate base.
- HC2H3O2 + H2O ⇌ H3O+ + C2H3O2-
- NH3 + H2O ⇌ NH4+ + OH-
- HNO3 + H2O ⇌ NO3- + H3O+
- Find the [H3O+] and pH of the following:
- 0.025 M HNO3
- 3.4 x 10-4 M H2SO4
- Calculate the [H3O+] and pOH of the following solutions:
- pH = 3.2
- pH = 5.0
- [OH-] = 8.2 x 10-9
- pH = 12.4
- Find the [OH-] of the following solutions:
- [H3O+] = 3.9 x 10-6 M
- [H3O+] = 0.0014 M
- pH = 4.2
- If 35.2 mL of 12M HCl is used to titrate 50.0 mL of KOH, what is the concentration of the KOH?
- What is the volume of 2.5 M NaOH needed to titrate 25.0 mL of 1.0 M HCl?
- Given the following equation, find the concentration of NaOH when 24.09 mL of 1.605 M H2SO4 is needed to titrate 50.0 mL of NaOH. H2SO4 + 2NaOH ➝ Na2SO4 + 2H2O
- What is the salt that is formed during the following neutralization reactions?
- Mg(OH)2 + HCl ➝ ? + H2O
- H2SO4 + 2NH4O ➝ ? + 2H2O
- Ni(OH)2 + 2HClO4 ➝ ? + 2H2O
- Mg(OH)2 + H2SO4 ➝ ? + 2H2O
- Balance the following redox reactions:
- Na + H2O → NaOH + H2
- HCl + HNO3 → HOCl + NO + H2O (*Cl on the product side has an oxidation number of +1)
- SnCl4 + Fe → SnCl2 + FeCl3
- CO + I2O5 → I2 + CO2
- MnO4- + Fe2+ + H+ → Fe3+ + Mn2+ + H2O
- Cu+ + Fe → Fe3+ + Cu
Tuesday, May 20, 2014
Practice for Final Exam
Tuesday, May 6, 2014
Proteins and Nucleic Acids
Proteins
Amino Acids
Polypeptide Chains
Proteins
- Most important substance in the bodies of living things.
- Building blocks for muscle, hair, blood cells, skin, slk, enzymes, insulin, etc.
Amino Acids
- Building blocks of proteins.
- Contain an amine (NH2) group and carboxyl (COOH) group.
- Various side chains (R groups) attach to the carbon adjacent to the N.
- Results in 20 different common amino acids.
- Our bodies can synthesize 12 out of 20.
- The other 8 must come from diet
- Known as essential amino acids
 |
| List of Amino Acids |
Polypeptide Chains
- Amino acids joined together with peptide bonds.
- Dipeptides: two amino acids joined together
- Aspartame (artificial sweetener)
- Polypeptides: polymers of many amino acids
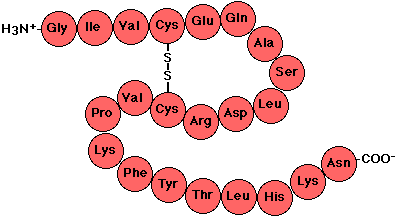 |
| Polypeptide chain or protein |
Proteins
- Polymer of one or more polypeptide chains.
- Some contain several hundred amino acids others several thousand.
- Various structures
Nucleic Acids
 |
| DNA Structure |
- When cells reproduce, they pass genetic information to one another in long-chain molecules called chromosomes.
- Human body contains 46 chromosomes in the nuclei.
- Segments of chromosomes, called genes, carry the coded information to that directs the production of specific polypeptide chains.
- Nucleotides are the building blocks of nucleic acid.
- Three units:
- 5 carbon sugar (ribose or dioxyribose)
- phosphate group
- ring-shaped nitrogen containing base
- There are five possible nitrogen-congaing bases attached to the sugar:
- Adenine (A)
- Cytosine (C)
- Guanine (G)
- Thymine (T)
- Uracil (U)
- DNA has A, C, G, T
- RNA has A, C, G, U
- DNA is two strains coiled around each other in a double helix
- When cells divide, a DNA strand reproduces by first unraveling with the help of enzymes.
- Each of the two resulting strands serves as a template, or pattern, for a new complementary chain.
- Complementary bases on the newly forming strand match the now-exposed bases of the old strand.
- When the process of replication is completed, two identical double-stranded DNA molecules result.
 |
| DNA Replication |
- One strand goes to each half of the dividing cell.
- DNA and RNA molecules instruct cells on how to make proteins.
- DNA is used as a template to make RNA.
- RNA is made up of nucleotides.
- Trinucleotide groups (3 nucleotides) code for various amino acids.
- As the amino acids join together, they form proteins.
- The proteins are the chemical manifestation of our physical characteristics.
 |
| This is an example of protein synthesis from RNA |
Differences between DNA and RNA
- DNA
- the sugar is deoxyribose
- contains thymine
- double strand
- RNA
- the sugar is ribose
- contains uracil
- single strand
Carbohydrates
- Most abundant biological compounds.
- Sugars and starches make up a large part of the human diet.
- Each year photosynthetic processes in plants convert water and carbon dioxide into one hundred billion tons of carbohydrates.
- Exoskeletons of insects are made of carbohydrates.
- All carbohydrates have several of the –OH (hydroxy) groups common to alcohols.
- They also have the C=O (carbonyl) group of aldehydes and ketones.
- They are known as one or more polyhydroxy aldehyde or ketone
- Three main functions:
- energy storage
- an energy source for cellular functions
- glucose
- structural elements in plants and animals
- cellulose & chitin
- Classified into three groups based on the number of sugar units they contain.
- Monosaccharides
- Disaccharides
- Polysaccharides
Monosaccharides
- These are simple sugars. One polyhydroxy aldhyde or ketone.
- Rarely occur in nature by themselves.
- Usually bonded to an protein, fat or other carbohydrate.
- Glucose is the most abundant sugar in nature.
- The human body maintains a reasonably constant level of 80 to 120 mg of glu- cose per 100 mL of blood.
- Glucose is also the fundamental building block of the most common long-chain carbohydrates.
- Glucose in an aqueous solution exists in an equilibrium between the ring form and the straight-chain form.
- Fructose also forms ring structures when it is in solution.
- Since the carbonyl group is not at the end of the carbon chain, it forms a five-member ring.
Disaccharides
- Contain two monosaccharide units.
- An oxygen bridge between the two monosaccharides holds the two units together.
- Three disaccharides play an important part in the human diet—maltose, lactose, and sucrose.
- Maltose (glucose - glucose)
- germinating grain & digestion of starches
- Lactose (glucose - galactose)
- milk
- Sucrose (glucose - fructose)
- table sugar, fruits, sugar cane, beets, nectar
Polysaccharides
- Many sugar units, up to several million units
- Can be built with several different monosaccharide units
- Glycogen: energy storage
- Cellulose: gives plants structure
- Starch: supply nearly 3/4ths of the worlds food energy
Subscribe to:
Comments (Atom)